| O’Brien, et.al., have demonstrated the self-assembly of molecularly thin, continuous fibrin sheets in situ from physiologic buffers having normal pH and ionic strength. The naturally occurring fibrin polymerization, and subsequent ability to self-associate to form extended 2-D molecular scale sheets, represents a new class of biological membrane. |
Reviewed by Jeff Morse, Ph.D., National Nanomanufacturing Network
The polymerization and characteristics of fibrin formation have been studied for decades due to the key role fibrin plays in blood clotting during the healing of wounds, as well as the formation of pathogen vascular thrombi. The parent protein to fibrin, fibrinogen, can have specific binding sites exposed through the cleavage and removal of small peptide components by the enzyme thrombin. This then enables the binding of corresponding regions between neighboring fibrin molecules. Through this process it is possible for fibrin to self-associate to form elongated, highly branched fibers and fiber networks. These fibrin fibers typically are elastic in nature, with good adhesive properties, and exhibit very high extensibility. Combining these fibers with platelets and blood cells will readily form clots in response to vascular injury.
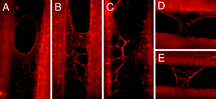
Early research investigated the formation of fibrin films in compressed clots as one viable means to generate a biological membrane polymer that could be used for controlled blood clotting. More recently, O’Brien, et.al., have demonstrated the self-assembly of molecularly thin, continuous fibrin sheets in situ from physiologic buffers having normal pH and ionic strength. The naturally occurring fibrin polymerization, and subsequent ability to self-associate to form extended 2-D molecular scale sheets, represents a new class of biological membrane.
The process by which these molecular sheets form occurs without external manipulation and is relatively spontaneous on both flat and textured surfaces. The authors describe self-assembly of continuous fibrin sheets conducted on micropatterned structured surfaces having 25 µm trenches formed in glass substrates. The substrate was placed in a dilute buffer solution containing low concentration (nanomolar) of fibrinogen and thrombin. Microscopic observation of the self-assembly process showed rapid polymerization of fibrin sheets capable of bridging across the surface structures and features formed on the substrate. Furthermore, the spontaneous nature of the polymerization process demonstrated extremely large rate constants in comparison to other protein polymers, on the order of 100’s of µm2/sec. Other aspects of the self-assembled fibrin sheets studied include the ability to form continuous sheets—and the relationship of tension forming within the sheets to the generation of fiber branching—the exposure of gaps within the sheets, and methods for controlling and better understanding the spontaneous processes forming these sheets.
While there remains a range of questions to be investigated to better understand the fibrin sheets' materials properties and gain control over their self-assembly characteristics, the inherent nature of the formation processes represents a significant step towards scaled nanomanufacturing of novel biological membranes. The reproducible nature of these fibrin sheets along with the relatively simple procedures by which the sheets can form on prepared surfaces can be directed towards medical applications for wound healing and vascular injury therapeutics. Furthermore, the molecular scale sheets offer a unique methodology to create and study both the materials properties and the kinetics of formation for these highly unique films.
Image source O'Brien, E.T., Falvo, M.R., Millard, D., Eastwood, B., Taylor, R.M., Superfine, R., "Ultrathin self-assembled fibrin sheets," PNAS 105 49 (2008) 19438-19443. Copyright PNAS 2008.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported.
