| Magasinski and colleagues report a potentially versatile method for synthesizing complex, high surface area composite structures for Lithium-Ion battery anodes incorporating bottom-up and top-down methods. |
Reviewed by Jeff Morse, Ph.D, National Nanomanufacturing Network
To address future needs for automotive and grid-scale energy storage, transformational pathways to increase the capacity for rechargeable batteries are necessary in order to reduce battery weight, size and cost. Silicon (Si) based materials for lithium-ion (Li+) battery anodes have received increased attention recently due to their potential for an order of magnitude increase in specific capacity in comparison to conventional graphite materials. While the low discharge potential and high theoretical capacity for silicon are extremely attractive, the key challenge has been to retain the Si anode capacity during battery cycling. The basis for this problem is the large volume change in the Si during Li+ insertion and extraction, leading to loss of electrical connection and ultimately electrode failure.
One method to address this issue includes the use of silicon nanoparticles mixed with polymeric binders; however this approach has not yet shown performance improvements. Another method uses Si nanowires grown on metallic substrates. The small diameter of the nanowires enables more favorable Li+ kinetics for improved charge/discharge performance, but the approach suffers from lack of control over nanowire morphology. This method, coupled with production-scale issues such as nanowire adhesion, synthesis costs, and cell assembly, will require significant development to become competitive.
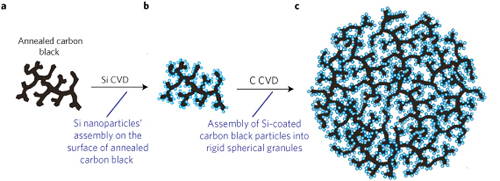
The porous Si-C synthesis begins with annealed carbon black, which forms a multi-branched dendritic nanoparticle 0.5-1.0 µm in size. Silicon nanoparticles are then coated on the carbon by chemical vapor deposition (CVD) using silane gas as the reactant. The Si nanoparticle size and density is controlled by CVD time, temperature, and pressure, with the pore size for the composite determined by the silicon nanoparticle size and the size of the branches in the initial C structure. Finally, a carbon CVD process is conducted on the Si-carbon composite resulting in the formation of rigid spherical granules having both high surface area and high porosity, thereby enabling ion accessibility to the Si anode materials during charge/discharge cycles. The carbon CVD step ,using propylene as the reactant gas, results in the granulation of the initial Si-C composite particles through a controlled self-assembly process. The Si-C anode performance can be further optimized through control of the nanostructured composite properties prior to the carbon CVD self-assembly step. Additional attributes of the process include scalability, low cost, and elimination of airborne nanoparticles improved environmental safety.
Electrochemical testing of coin cells using a Li metal counter electrode exhibited high capacity for the Si-C composite anode structure, exhibiting ~1500 mAh/g for 100 charge/discharge cycles. Additionally, the Si-C composite anode exhibited significantly higher rate capacity in comparison to conventional graphite anodes, approximately 37 times higher at C/20 capacity at a rate of 2.98 A/g. The authors theorized that these combined attributes of the Si-C composite anode were a result of the silicon nanoparticle coated pore structure providing structural stability during Si volume change, good ion accessibility, and good electrical conductivity. Furthermore, this approach can be applied to cathode materials for further optimization of both electrode structures. Thus, a potentially versatile method has been reported incorporating bottom-up and top-down methods to synthesize complex, high surface area composite structures for a range of potential applications.
Images reproduced from Macmillan Publishers Ltd: Magasinski Nature Materials 9(2010): 353 - 358. DOI: 10.1038/nmat2725. Copyright 2010.
This work is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported.
