| Stepanow et. al. reported on the fabrication of ordered 2D nanostructures in a solvent-free, high vacuum environment. In this work the authors selected several carboxylic acid groups and alkali metal atoms as the building blocks for supramolecular assembly. This approach potentially represents a facile, versatile method for the fabrication of highly ordered 2D nanostructures via a bottom up ionic assembly. |
Reviewed by Jeff Morse, PhD., National Nanomanufacturing Network
Fabrication of ordered nanostructures via bottom up self-assembly techniques has gained widespread interest for a range of applications due to the potentially facile process approach in comparison to multistep lithographic methods. Approaches exploiting electrostatic interactions for the self-assembly of oppositely charged nanostructures have become an effective tool to fabricate nanostructured materials with specific morphologies having unique structural and chemical properties. Examples of electrostatically-controlled assembly include field directed and layer-by-layer approaches that enable orientation of functionalized nanostructures on surfaces. Another method employs the supramolecular assembly concept in which intermolecular interactions, such as hydrogen bonds, van der Waals forces, and surface properties, are coordinated to link specific building blocks. In this manner, the interactions between the polar regions of a nanostructure can be used to steer the molecular organization. Such ionic self-assembly methods have the advantages of process simplicity and versatility, and can utilize a wide range of building block components. A disadvantage of this approach is the wet chemistry environment resulting in limited assembly rates and space charge effects. As such, this approach has had limited success for the fabrication of two-dimensional (2D) ordered nanostructures.
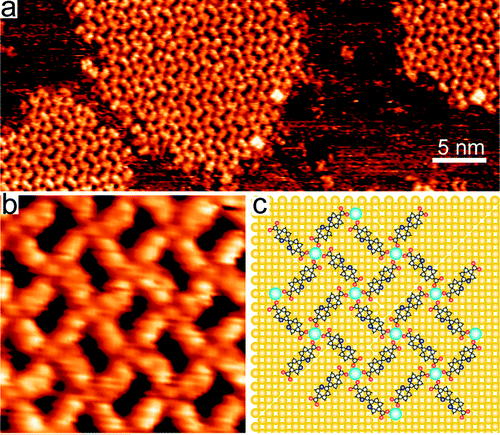
The authors used cesium (Cs) adatoms as the oppositely charged component for the electrostatic assembly. An alkali metal dispenser was used to controllably deposit a sub-monolayer of Cs on the Cu surface resulting in a lowering of the work function enabling charge transfer between the Cs and Cu substrate. In this manner the adsorbed Cs atoms become the cations providing a repulsive force between adjacent atoms preventing formation of metal clusters while providing a complimentary component to the linker molecules having carboxylate anions. These components thereby facilitate an ionic attraction on the metal surface. Using this technique the authors were able to steer the molecular organization of the carboxylate moieties and Cs adatoms on the Cu surface to fabricate well-defined 2D structures. Once the Cs atoms were deposited onto the Cu surface, ordered structures self-assembled into various morphologies.
In this study the authors found that with the appropriate concentration and ratio of organic linker molecules and Cs adatoms, the mixture assembles into open 2D square patterns having characteristic dimensions associated with the aromatic backbone length (7-16 Angstroms) as determined by scanning tunneling microscopy. The authors further utilized ab initio models to better understand and verify the ionic assembly phenomenon. From the combined experimental and theoretical results, the authors were able to better understand the impact of metal surface properties, along with concentrations of organic linker and alkali metal components in order to improve the control of the assembly. Future investigations will consider broadening the range of surfaces and components in forming organized 2D nanostructures in order to better control the balance of adsorption strength and electrostatic interactions. This approach potentially represents a facile, versatile method for the fabrication of highly ordered 2D nanostructures via a bottom up ionic assembly.
Image reproduced with permission from Stepanow S, et al. 2010. ACS Nano 4(4): 1813–1820. DOI: 10.1021/nn100303z. Copyright 2010 American Chemical Society.
This work is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported.
