New approaches to nonvolatile memory devices have previously been demonstrated by charge trapping on metallic nanoparticles (NPs) embedded in organic layers. Devices based on this concept have shown that the memory capability of the device is affected by the distribution of the NPs. In the case of gold nanoparticles (AuNPs) that are frequently embedded in the organic layer or on the surface, the migration of the AuNPs during operation results in limited stability and device lifetime. Furthermore, the origin of the AuNP charging effects is not completely understood. One scheme to better stabilize the AuNPs while further ensuring effective charging conductive paths and simultaneously providing a barrier to discharging is the use immobilized AuNPs on semiconducting reduced graphene oxide (rGO) nanosheets.
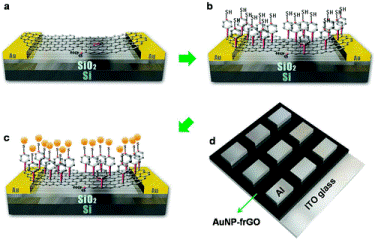
Once the rGO nanosheets layer was formed between the gold contacts, a monlayer of AuNPs were chemically bound to the rGO through a π-conjugated molecular linker. In this approach, 4-Mercapto-benzenediazonium tetraflouroborate (MBDT) salt was designed to have bifunctional groups with one end being a diazonium salt that can be chemically bonded to rGO, and the other end being a thiol group that can spontaneously attach to AuNPs. To assist in the charge transport onto the AuNPs, the simplest π-conjugated aromatic group, phenyl, was selected as a communicative wire. The synthesized molecular linker further provides an energy barrier to charge transport, thus the charge can be held by the nanoparticles for effectively long times. Devices were fabricated in both horizontal and vertical configurations. Spectral analysis characterization of the resulting layers in confirmed the chemical bonding between the rGO nanosheets and the AuNPs. Voltage scanning of the devices exhibited consistently reproducible and nonlinear hysteresis behavior suitable for memory devices. Devices were further subjected to write-multiple read-erase-multiple read characterization, exhibiting excellent stability and performance during these operations. Studies of horizontal memory device layouts demonstrated that the charging effect does in fact originate from the AuNPs. Additionally, current-voltage characterization of both the horizontal and vertical device designs exhibited excellent stability and reproducibility, as well as long charge retention times (>700 seconds). Finally, the vertical AuNP-functionalized rGO memory devices exhibited large On/Off ratios suitable for nonvolatile memory applications. Thus a bottom up approach incorporating a new method to chemically bond nanostructured materials provides enhancements in both device performance and stability.
Reviewed by Jeff Morse, PhD, National Nanomanufacturing Network
- Cui P, Seo S, Lee J, Wang L, Lee E, Min M, Lee H. 2011. Nonvolatile memory device using gold nanoparticles covalently bound to reduced graphene oxide. ACS Nano. 5(9): 6826-6833. http://dx.doi.org/10.1021/nn2021875
Figure reprinted by permission from American Chemical Society: Cui P, Seo S, Lee J, Wang L, Lee E, Min M, Lee H. 2011. Nonvolatile memory device using gold nanoparticles covalently bound to reduced graphene oxide. ACS Nano. 5(9): 6826-6833. http://dx.doi.org/10.1021/nn2021875
