First Clinical Program for Selecta's Synthetic Vaccine Particle Platform with Broad Potential for a Range of Therapeutic Applications Including Infections, Cancer, Allergies, and Autoimmune Diseases 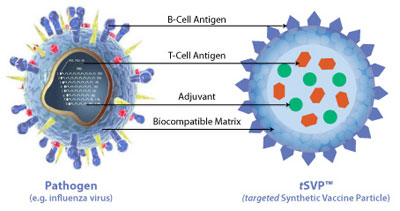
"This is the first time ever that a fully integrated synthetic, nanoparticle vaccine is being tested in human clinical trials and is a very important milestone in the translation of Selecta's SVP technology," said Ulrich von Andrian, Ph.D., M.D., Edward Mallinckrodt Jr. Professor of Immunopathology at Harvard Medical School and Selecta co-founder. "Selecta has demonstrated its ability to rationally design immunotherapeutics and induce a robust targeted immune response. SVP technology will revolutionize the way vaccines will be designed, produced and applied."
The Phase 1 clinical study of SEL-068 is a double-blind, placebo-controlled, ascending dose study in healthy, non-smoking and smoking volunteers. In addition to safety, the study will evaluate the vaccine's potency through the measurement of concentrations of nicotine-specific antibodies. Selecta expects to report initial results from this Phase 1 study in the first half of 2012.
Because SEL-068 is fully synthetic, the immune response is entirely focused on nicotine and avoids off-target responses to biological carriers typically used with other vaccine technologies. The resulting high antibody concentrations induced by SEL-068 have the potential to absorb inhaled nicotine, preventing it from reaching the brain and triggering the addictive response.
"I'm extremely pleased with the pre-clinical results we have obtained with our SVP technology, and I expect SEL-068 to meet the requirements for a successful smoking cessation and relapse prevention vaccine. By entering into Phase 1 human clinical studies, Selecta has demonstrated its capability to scale production and demonstrate safety in toxicology studies," said Werner Cautreels, Ph.D., President and CEO of Selecta. "We can now accelerate the development of our platform and bring forward our candidates in autoimmune, infectious diseases, and cancer."
Selecta's SEL-068 development program is funded in part with a grant from the National Institute of Drug Abuse (NIDA), an institute within the U.S. National Institutes of Health (NIH).
About Selecta
Selecta Biosciences, Inc. is a biopharmaceutical company developing an entirely new class of targeted vaccines that induces an antigen-specific immune activation or antigen-specific immune tolerance for therapeutic and prophylactic applications. Selecta was founded based on complementary research by three academic pioneers, the technology innovations of Professors Robert Langer and Omid Farokhzad combined with the immunological insights of Professor Ulrich von Andrian. Selecta's proprietary Synthetic Vaccine Particle (SVP™) platform creates a new paradigm in vaccine development, enabling completely new therapeutic and prophylactic applications while offering the potential of improved efficacy and safety profiles. Selecta's fully synthetic engineering of novel vaccines offers a number of compelling benefits, including flexible modular vaccine design and accelerated development timelines using robust manufacturing processes. Selecta's SVP™ platform technology is readily adaptable to enable diverse vaccines and the company has created antigen-specific targeted Synthetic Vaccine Particles (tSVP™) and antigen-specific targeted tolerogenic Synthetic Vaccine Particles (t2SVP™).
Targeted Synthetic Vaccine Particles (tSVP™) activate immune responses to a wide array of relevant antigens, including small molecules, peptides, oligosaccharides, and proteins. These particles can target humoral or cellular pathways of the immune system. Examples for applications include cancer, infectious diseases and addiction.
Targeted tolerogenic Synthetic Vaccine Particles (t2SVP™) are designed to induce antigen-specific immune tolerance. Examples for applications include autoimmune diseases, allergies, and transplant rejection.
Selecta's pipeline currently contains a vaccine for smoking cessation and relapse prevention, a vaccine for Type 1 Diabetes, vaccines for several infectious diseases (such as universal influenza and malaria), and research approaches with cancer vaccines and in the field of allergies.
Building on the company's novel approach, Selecta's product candidates have the potential to become first-in-class or best-in-class therapeutics to treat and prevent diseases. Selecta Biosciences, Inc. is based in Watertown, Massachusetts, USA. For more information, please visit www.selectabio.com.
Source: Selecta Biosciences
