Stimulus-responsive biomaterials are of significant interest as in vivo drug delivery systems. Such materials provide a means for controlled and long-term drug release as new treatments for a range of chronic diseases that require daily injections or precise doses of specific medications. Various materials systems investigated to date have exhibited response to heat, light, pH, enzymes, ultrasonic waves, and magnetic fields. While some interesting performance has been reported utilizing these stimulus methods, activation of these materials typically requires large or specialized equipment. In comparison, electric-field stimulus is much simpler to generate and control. Electrical signals have been shown to release molecules via conducting polymeric bulk materials or implantable electronic delivery devices, yet often require invasive surgery to implant and activate the devices. In order to implement electrically activated drug delivery, a technique is required that encapsulates the drug compound in a platform suitable for injection to a specific locale where the release can be triggered.
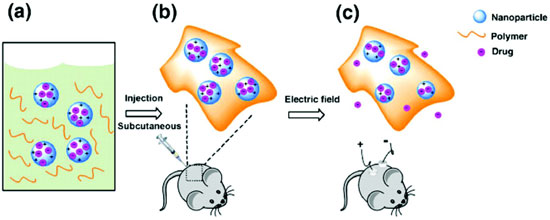
Recently, Ge et. al. reported on an investigation of electric-field responsive nanoparticles for drug release. In their study, the authors utilized emulsion polymerization methods to encapsulate drug compounds in conductive polypyrrole (PPy) nanoparticles that are suspended in a hydrogel. The temperature sensitive hydrogel, which is a liquid at low temperature, and can be tailored to become a gel at body temperature, is used to localize the PPy nanoparticles after they are subcutaneously injected at the point of interest. The hydrogel further provides a biodegradable and biocompatible matrix for the conductive nanoparticles. The nanoparticles provide additional features including increased surface area for high drug loading, along with the small size (50-100 nm) that enables ease of passage and excretion through the circulatory system. Flourescein and daunorubicin compounds were selected to be loaded into PPy nanoparticle during synthesis, which after polymerization were completely encapsulated. Nanoparticles with each compound exhibited similar morphologies with average diameters of ~60 nm. PPy nanoparticles were then suspended in a hydrogel formulation designed such that the solution was a liquid at low temperatures, and the critical gelation temperature occurred at higher temperature (body temperature).
The authors first studied the drug release characteristics in a phosphate buffered saline solution. Two platinum electrodes were placed in the solution 1 cm apart, with one of the electrodes coated with a 1mm thick hydrogel solution containing 0.25 wt% fluorescein-encapsulated PPy nanoparticles. An electrical signal of 0.5 V was applied for 10 seconds followed by measurement of the concentration of free fluorescein in the solution. This was repeated every 5 min. Over a 30 min period, ~20 ng of fluorescein was released for each electrical pulse. Increasing the electrical pulse to 1.5 V, the authors observed an initial release of ~60 ng, with each subsequent release being ~ 30 ng. Similar results were observed for daunorubicin encapsulated NPs. Furthermore, long-term release studies were conducted in which the stimulus pulse was applied once every 24 hours, and showed similar results. Additional control studies showed that now drug compounds were released without electrical pulses, and that drug compounds in the hydrogel alone without encapsulation were readily released into the solution with no control. The mechanism behind the electric-field release is believed to be associated with an electrochemical reduction/oxidation process that is known to occur in bulk PPy. These reactions result in a net charging of the PPy nanoparticle which provides the repulsive force to release the molecules with application of the electrical pulse. Subsequent electric-field drug release was conducted in vivo on mice with nanoparticle containing hydrogel solution injected into two sites. Electric-field stimulus was applied to one site, and the other site was left as a control with no signal applied. The fluorescein was monitored by fluorescent imaging of each site. Results showed an increase in the fluorescein signal after each release, with no obvious release at the site with no field applied. Thus a facile, minimally invasive approach to controlled drug delivery has been demonstrated using electrically stimulated conducting PPy nanoparticles. The approach described can potentially be broadly used to trigger ultra-sensitive dosage controlled drug release, and further has unprecedented spatial and temporal control.
Reviewed by Jeff Morse, PhD, National Nanomanufacturing Network
- Ge J, Neofytou E, Cahill TJ, Beygui RE, Zare RN. 2011. Drug release from electric-field-responsive nanoparticles. ACS Nano. 6(1): 227-233. http://dx.doi.org/10.1021/nn203430m
Figure reprinted with permission from Ge J, Neofytou E, Cahill TJ, Beygui RE, Zare RN. 2011. Drug release from electric-field-responsive nanoparticles. ACS Nano. 6(1): 227-233. http://dx.doi.org/10.1021/nn203430m. Copyright 2011 American Chemical Society.
