Graphene is easy to acquire, at least in small amounts. The first scientists to isolate the strong, two-dimensional carbon material simply pressed a piece of Scotch tape to a chunk of graphite and peeled it off. But mass production of graphene for commercial uses remains a challenge. Now, scientists have shown they can rapidly produce large quantities of graphene using a bath of inorganic salts and an electric current (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja5017156).
Several other methods have been developed for producing graphene, but each has its drawbacks. Growing the carbon sheets takes too long, and chemical vapor deposition requires a metal catalyst, with a second step to remove the metal. Other methods using solvents or surfactants can harm the electronic properties of graphene or produce lower yields.
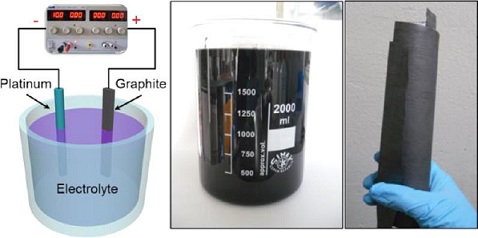
Xinliang Feng and Klaus Müllen of the Max Planck Institute for Polymer Research, in Mainz, Germany, and their colleagues decided to improve upon an electrochemical technique for producing graphene. Instead of using acids, which oxidize the graphene and reduce its conductivity, the researchers prepared solutions of various salts, including ammonium sulfate, potassium sulfate, and sodium sulfate. Into their mixtures they placed two electrodes, one made of platinum and the other of graphite, which is essentially a conglomeration of many layers of graphene. When they ran 10 V of direct current through the graphite electrode, it began to shed layers into the solution, a process called exfoliation. They kept the current running for three to five minutes, separated the exfoliated flakes from the solution, and washed away excess salt with water.
The process turned more than 75% of the graphite electrode into graphene flakes. Approximately 85% of the flakes consisted of one to three layers of graphene—the most desirable electrical properties come from single and double layers of graphene.
Of the solutions they tested, the ammonium sulfate worked the best, producing the highest quality graphene in the fastest time. In one test, the researchers were able to produce approximately 16.3 g of graphene in 30 minutes. But Feng sees the potential to scale up production to the kilogram scale needed for industrial use.
James M. Tour, a synthetic organic chemist at Rice University, calls the work “very nice—not the first time such electrochemical exfoliation has been done, but the authors here get it to work more efficiently.”
The exfoliation process is also more environmentally friendly than previous methods for generating graphene, Feng says, and doesn’t require high temperatures. He plans to test other electrolytes as well as different forms of graphite in an effort to scale up the process. The individual sheets of graphene, however, are still very small, at most a few millimeters across. Producing sheets on the centimeter scale, to build something like a transparent electrode, remains difficult, Feng says.
To demonstrate a use of their graphene, the team mixed the powder they produced into N,N’-dimethylformamide to produce a graphene ink, which they painted in a thin film on a piece of paper. They used two pieces of treated paper to make a supercapacitor, showing the material’s potential use in flexible electronics.
Lain-Jong Li, a research fellow at Academia Sinica, in Taiwan, says his group has developed a similar process and started a company, Nitronix, which hopes to produce 100 tons of graphene annually within two years. Feng, meanwhile, is working with the German chemical company BASF to scale up their production.
Source: Chemical & Engineering News
