| Email not displaying correctly? View it in your browser. |
 |
December 7 2011 |
|
Selecta Biosciences Initiates Phase 1 Clinical Study of SEL-068, a First-in-Class Synthetic Nicotine Vaccine for Smoking Cessation and Relapse Prevention
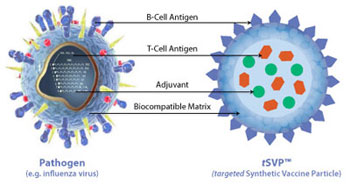
Selecta Biosciences, Inc., a biopharmaceutical company developing a new class of synthetic vaccines and immunotherapies, today announced that it has initiated a Phase 1 clinical trial to assess the safety, tolerability and pharmacodynamic profile of SEL-068, a nicotine vaccine candidate for smoking cessation and relapse prevention. SEL-068 is the first product candidate to enter clinical evaluation from Selecta's proprietary Synthetic Vaccine Particle (SVP™) Platform, and has the potential to become the first nanoparticle vaccine that is synthetically engineered, distinct from conventional biological vaccine manufacturing processes. "This is the first time ever that a fully integrated synthetic, nanoparticle vaccine is being tested in human clinical trials and is a very important milestone in the translation of Selecta's SVP technology," said Ulrich von Andrian, Ph.D., M.D., Edward Mallinckrodt Jr. Professor of Immunopathology at Harvard Medical School and Selecta co-founder. "Selecta has demonstrated its ability to rationally design immunotherapeutics and induce a robust targeted immune response. SVP technology will revolutionize the way vaccines will be designed, produced and applied." The Phase 1 clinical study of SEL-068 is a double-blind, placebo-controlled, ascending dose study in healthy, non-smoking and smoking volunteers. In addition to safety, the study will evaluate the vaccine's potency through the measurement of concentrations of nicotine-specific antibodies. Selecta expects to report initial results from this Phase 1 study in the first half of 2012.
|
Join Us In The Library Nano/bio Integrations for Biosensing and Drug Delivery Going On 2011 NSF Nanoscale Science and Engineering Grantees Conference International Workshop on Challenges to Increased Use of Nanotechnology Standards International Conference on Nano Science and Technology Submit International Conference on Nano Science and Technology International Conference on the Chemistry of Nanotubes and Graphene Nanotech 2012 Conference and Expo |
||||
|
Subscribe / Unsubscribe from this list. Our mailing address is: The National Nanomanufacturing Network 374 Lederle Graduate Research Center 710 N. Pleasant Street University of Massachusetts Amherst, MA 01003 Our email address is: nnn@nanomanufacturing.org Our phone number is: (413) 577-0570 Copyright (C) 2011 The National Nanomanufacturing Network All rights reserved. Supported by the National Science Foundation under Grant No. CMMI-1025020. |
|||||