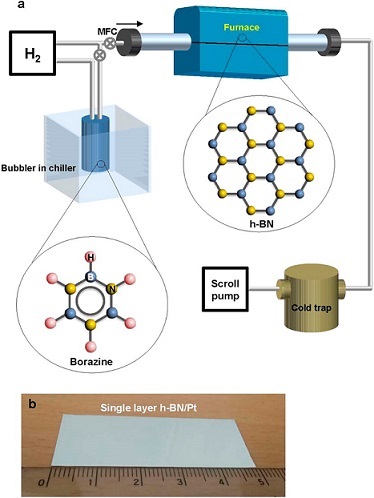
Recently, Park et.al., reported results from a systematic study for synthesis of large area single layer h-BN films on polycrystalline Pt foils using low pressure CVD comparing borazine and ammonia borane precursors. The authors’ goal was to study the effect of the Pt lattice orientation, the total pressure, and the different cooling rate in order to understand h-BN growth mechanisms. Since nitrogen is not soluble in Pt, the authors’ objective was to confirm the contributions to h-BN growth surface mediated and precipitation processes. The study included analysis of film properties dependence on cooling rate and crystal orientation of the substrate. Their findings demonstrated that film growth was by a surface mediated growth mechanism, facilitated by a catalytic reaction, that produced polycrystalline h-BN monolayers confined by the underlying Pt surface orientation. The thickness of the h-BN films exhibited a dependence on the Pt surface orientation, presumably determined by the available catalytic reaction sites that decompose the borazine precursor, which would exhibit a dependence on crystal orientation.
Improved understanding of h-BN growth mechanisms will potentially lead to methods for controlling the growth of high-quality h-BN films. This further provides the basis for materials and substrates for application in quantum tunneling devices, novel heterostructures, and two-dimensional semiconductors such as molybdenum sulfide and graphene.
Reference: Park J, Park JC, Yun SJ, Kim H, Luong DH, Kim SM, Choi SH, Yang W, Kong J, Kim KK, Lee YH. Large-Area Monolayer Hexagonal Boron Nitride on Pt Foil. ACS Nano. 2014; 8 (8): 8520-852 doi: 10.1021/nn503140y
Image reprinted with permission from American Chemical Society.
