| Ji et. al. investigate the use of nanostructured sulphur/mesoporous carbon as a cathode material to overcome the challenges of Lithium-Sulfur cell technology, suggesting a path to the realization of high-capacity, long-cycle-life rechargeable batteries. |
Reviewed by Jeff Morse, Ph.D, National Nanomanufacturing Network
Energy storage systems for renewable and regenerative power sources have focused on low-cost, high-energy-density rechargeable battery technologies. Present lithium ion cell (Li+) technology operates via a lithium transition metal-oxide or phosphate cathode and carbon-based anode separated by an electrolyte facilitating the intercalation and transport of Li+ though the charge/discharge cycles. These specific materials systems have inherent limitations in charge storage capacities on the order of 300 mAh/g, with maximum capacities reported on the order of 180 mAh/g. Furthermore, the range of chemistries being investigated for rechargeable Li+ batteries have specific benefits and limits dependent on materials systems, including maximum capacity, charge/discharge rate, safety, and cycle lifetime. Although a range of technologies and chemistries are commercially available, to date there has been no specific technology demonstrating superior performance for all applications.
A promising technology for energy storage that has been studied for many years is the Lithium-Sulphur (Li-S) cell, which operates via a redox with sulphur as the positive electrode and lithium as the negative electrode. While the reaction potential is only 2.2 volts for Li-S cells—significantly less than that exhibited by conventional positive electrodes—the theoretical capacity is on the order of 1675 mAh/g, potentially enabling rechargeable batteries with much higher gravimetric or volumetric energy density.
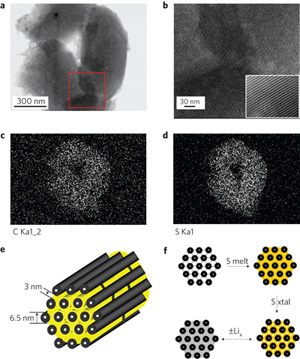
Recently, Ji et. al. investigated the use of nanostructured sulphur/mesoporous carbon as a cathode material to overcome these challenges. Using CMK-3—a type of mesoporous carbon exhibiting an interconnected pore structure with uniform pore diameter, high pore volume, and good electrical conductivity—the authors nanocasted replica structures of the mesoporous carbon yielding hollow 6.5 nm carbon rods with 3-4 nm channel spacing. The authors then created a CMK-3/sulphur composite via a simple melt-diffusion approach in which the sulphur is heated to just beyond the melting point, and wicks into the pore channels by capillary forces. Upon cooling, the sulphur solidifies, thereby forming sulphur nanofibers in intimate contact with the conductive carbon matrix. Test results for Li-S cells fabricated with the CMK-3/sulphur composite exhibited significant improvements in capacity to >1000 mAh/g.
The authors further investigated a method to prevent mass loss by polysulfide anion migration using polyethylene glycol (PEG) to functionalize the mesoporous surface after imbibing the sulphur within the pores in order to render the carbon surface hydrophilic, and further entrap the sulphur active mass. This approach not only improved the cell stability through multiple cycles, but also increased the initial discharge capacity of the cell to 1,320 mAh/g, approximately 80% of the theoretical limit.
This research suggests a path to the realization of high-capacity, long-cycle-life rechargeable batteries. Further investigation may consider other forms of mesoporous carbon, along with other materials that can be added to the pore structure for various applications. Additionally, the uniformity and reproducibility of this method must be demonstrated in larger scale processes.
Image reproduced by permission from Macmillan Publishers Ltd: Ji X, et al. 2009. Nature Materials 8(6): 500-506, copyright 2009.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported.
