| Zorn, et.al, describe a hybrid Quantum Dot-Block Copolymer system for LEDs that exhibits the excellent optical properties of the QD materials and maintains the processability of BCP systems. |
Reviewed by Jeff Morse, Ph.D., National Nanomanufacturing Network
- Zorn M,Bae WK,Kwak J, Lee H, Lee C, Zentel R and Char K.(2009) Quantum Dot Block Copolymer Hybrids with Improved Properties and Their Application to Quantum Dot Light-Emitting Devices. ACS Nano 3(5): 1063–1068. DOI:10.1021/nn800790s.
Quantum dot (QD) materials exhibit distinct physical behaviors compared to bulk materials as a result of size-dependent properties known as quantum confinement effects. The high fluorescence quantum yields and optical stability of inorganic QDs, for example, make them interesting nanomaterials for photovoltaics, light emitting diodes, or fluorescent sensors--with the ability to tune the fluorescence and emission properties over a broad spectral range simply by varying the particle size. QDs offer the benefits of high efficiency fluorescent inorganic materials as well as dispersion via solution processes. Block copolymer (BCP) systems are ideally suited to solution-based processes. Combining the desirable properties of these materials systems offers a powerful approach to coordinated self assembly of hybrid materials systems.
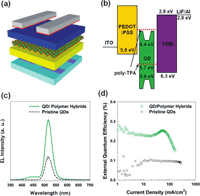
Zorn, et.al., report their investigation of QD-BCP hybrids for improving the properties of QD light emitting diodes (QLEDs). In this investigation, QD/polymer hybrids were created by grafting block copolymer containing thiol-anchoring groups (PTPA-b-CAA) onto the surfaces of CdSe/ZnS QDs via a ligand exchange process. Exhibiting the best of both worlds, the hybrid system exhibited the excellent optical properties of the QD materials, combined with the processability of BCP systems (IE: enhanced solubility in typical solvents, improved colloidal stability of the QDs, and excellent film forming ability).
The authors made a layered QLED test device to study the electroluminescence of their materials. Compared to QLED devices with unmodified QDs, the hybrid test device exhibited significantly increased electroluminesence intensity at similar operating points and a 3-fold increase in external quantum efficiency. The authors attribute these results to improved uniformity of the light emitting layer formed by the hybrid process, combined with improved hole injection into the QDs facilitated by the grafted polymer matrix.
This work has clearly shown the improved process capability incorporating cooperative self assembly methods for hybrid inorganic/organic material systems. Furthermore, the hybrid system has demonstrated new functionality not yet exhibited by either material system individually. This represents a major step towards long term goals for nanomanufacturing wherein the controlled manipulation of materials and structures can be conducted at the molecular scale with inherent process scalability. These approaches might be further extended to a range of nanostructures and materials, including rods, wires, tubes and sheets, for a range of future applications.
Image reproduced with permission from Zorn M,Bae WK,Kwak J, Lee H, Lee C, Zentel R and Char K.(2009) Quantum Dot Block Copolymer Hybrids with Improved Properties and Their Application to Quantum Dot Light-Emitting Devices. ACS Nano 3(5): 1063–1068. DOI:10.1021/nn800790s. Copyright 2009 American Chemical Society.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported.
