| Nejati and Lau investigate initiated chemical vapor deposition (iCVD) as a solvent-free method to form solid polymers in nanoscale structures, circumventing the challenges of replacing liquid electrolytes with solid state or gel materials in dye sensitized solar cells (DSSCs). |
Reviewed by Jeff Morse, PhD, National Nanomanufacturing Network
As dye sensitized solar cells (DSSC) have exhibited increased potential as a competitive photovoltaic technology for harvesting sunlight, the utilization of mesoporous titanium dioxide (TiO2) has played a significant role for increasing cell efficiency by providing the photosensitizing dye with greater surface area. A key limitation for DSSCs is the use of liquid electrolyte, which presents challenges for sealing and packaging, durability, and long-term robustness—highly important aspects for any technology to compete in the growing markets of renewable energy and solar photovoltaics. While many options for replacing liquid electrolytes with solid state or gel materials have been explored, infiltrating the mesoporous TiO2 with solid materials remains a significant challenge. Techniques for filling the pore structures have been limited to electrodes on the order of ~2 µm thick, and even then are difficult using liquid based techniques due to the nanoscale diameters and long tortuous pathways encountered. Additionally, liquid-based approaches typically introduce solvents to the system which are difficult to remove and can further deteriorate device performance.
Recently, Nejati et. al. have investigated initiated chemical vapor deposition (iCVD) as a solvent-free method to form solid polymers in nanoscale structures, circumventing these issues for DSSCs. iCVD is an adsorption-limited process that depends on the gas phase transport of monomers and activated initiators to the surface followed by a surface chemical reaction. In this manner, iCVD enables polymerization and deposition in a single step, providing both nanoscopic physical control and chemical control using synthetic pathways necessary for DSSC fabrication. 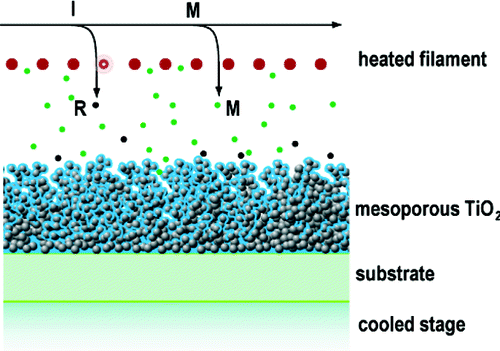
In this study, iCVD variables include total reactor pressure, monomer and initiator flow rates, and partial pressures. Characterization methods included scanning electron micrograph (SEM) analysis combined with thermogravimetric analysis (TGA). The authors observed that the pore filling was much more sensitive to the monomer than the initiator flow rate, and that a sweet spot existed for the total reactor pressure and reactant flow rates that completely filled the pore structures. In establishing a theoretical basis for their observations, the authors analyzed the limits of mass transport and reaction kinetics contributing to the pore filling processes. Within the sweet spot for complete pore filling, where the monomer partial pressure is in the 25-65 mTorr range (reactor pressure of 125 mTorr), the diffusion time constant is much less than the reaction time constant. As the process parameters are varied such that the reaction time constant dominates, incomplete surface reactions result in a polymer overcoating of the mesoporous TiO2 thereby preventing complete pore filling.
DSSC device characterization at standard illumination intensities demonstrated a higher open circuit voltage with similar short circuit current densities, thus the efficiency of the quasi-solid state electrolyte is higher than the liquid electrolyte counterparts. The authors attribute the increase in open circuit voltage exhibited by the solid electrolyte to the suppression of electron recombination at the electrolyte-electrode interface resulting from the complete pore filling through the iCVD process. Thus iCVD provides a means for coating and filling porous media having nanoscale features.
Image reproduced with permission from Nejati S and Lau KKS. 2010. Pore Filling of Nanostructured Electrodes in Dye Sensitized Solar Cells by Initiated Chemical Vapor Deposition. Nano Letters Article ASAP December 20, 2010. DOI: 10.1021/nl103020w. Copyright 2010 American Chemical Society.
This work is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported.
