| Carmo et. al. from Yale University recently reported on an approach to synthesize an electrode catalyst support using bulk metallic glass nanowires (BMGs). In this work, a top-down nanomanufacturing method is described to fabricate the Pt-BMG nanowire catalyst for electrochemical applications. The facile approach is scalable to large areas and further offers atomic level control over material composition and dispersion. Experimental results have indicated this approach may resolve key issues related to performance and durability for fuel cell applications, and may further impact additional energy generation and storage applications. |
Reviewed by Jeff Morse, PhD, National Nanomanufacturing Network
- Carmo M, Sekol RC, Ding S, Kumar G, Schroers J. Taylor AD. 2011. Bulk Metallic Glass Nanowire Architecture for Electrochemical Applications. ACS Nano 3 March 2011. doi:10.1021/nn200033c
Play the best slots online in Canada. We find, rank and rate the very latest slot releases in Canada.
The utilization of high surface area three-dimensional (3D) nanostructures provides significant performance enhancements for energy conversion and storage technologies such as fuel cells, batteries, and photovoltaics. In the case of fuel cells, traditional electrode catalyst supports incorporate carbon particles mixed with catalyst particles. Design for optimal electrode reactions requires a balance of catalyst distribution within a porous matrix to facilitate the energy conversion while providing sufficient electrical conduction through the carbon support. As a result, the catalyst utilization is typically quite low, which is problematic since catalysts are typically precious metals (e.g., Pt, Ru, Pd). While approaches to improve catalyst utilization and dispersion such as the use of carbon nanotubes decorated with catalysts have been successfully demonstrated, fuel cell durability remains an issue due to carbon degradation during operation resulting from loss of electrochemical surface area. To address these issues, a great deal of attention has been focused on nanostructured materials such as nanowires or nanotubes formed from metallic compositions.
Comparison of the electrochemical performance of the Pt-BMG catalyst support to a standard Pt/C support by cyclic voltammetry exhibited no loss of electrochemical surface area (ECSA) after 1000 cycles for the Pt-BMG versus 60% loss for the Pt/C support. The latter occurs due to degradation of the carbon support and resulting loss of Pt catalyst activity. In comparison, the Pt-BMG actually exhibits a slight increase in ECSA resulting from a dealloying of the BMG composition in which the least noble metal, Cy in instance, is selectively removed from the composition resulting in a higher effective surface area of accessible Pt catalyst. As such, this effect is beneficial for fuel cell applications. Additional benefits of the Pt-BMG catalyst composition include increased electrical conductivity in comparison to carbon supports, and increased tolerance hydrocarbon reaction intermediates, such as carbon monoxide, which can adsorb to the catalyst and reduce the overall electrochemical activity thereby poisoning the catalyst.
A top-down nanomanufacturing method has been described to fabricate the Pt-BMG nanowire catalyst for electrochemical applications. The facile approach is scalable to large areas and further offers atomic level control over material composition and dispersion. Experimental results have indicated this approach may resolve key issues related to performance and durability for fuel cell applications, and may further impact additional energy generation and storage applications.
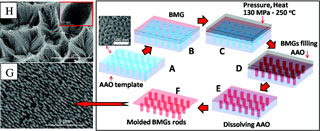
Image reproduced with permission from the American Chemical Society from Carmo M, Sekol RC, Ding S, Kumar G, Schroers J. Taylor AD. 2011. Bulk Metallic Glass Nanowire Architecture for Electrochemical Applications. ACS Nano 3 March 2011. doi:10.1021/nn200033c
This work is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported.
