Cobalt nanowires with high perpendicular magnetic anisotropy are formed in a diblock copolymer film template using a pulse-reversed voltage with QCM monitoring. This in situ monitoring system along with the pulse-reversed field enables new control over the magnetic crystal growth.
Andrei Ursache, Mark Tuominen, T. P. Russell
NSF Center for Hierarchical Manufacturing
A gold-coated 5 MHz AT-cut crystal with a PS template acts as the electrodeposition template.
An electrolyte solution consisting of deionized water, methanol, cobalt sulfate, and boric acid is formed. To maintain a pH of 6.0, sodium tetraborate is added.
With the sample in solution, two pulses are applied (-1.0 and -0.35 V) for 10 ms each. The intention is to remove atoms with low binding energies from the surface to maintain better crystal formation.
By monitoring the resonant frequency of the (Maxtek RQCM) QCM and electrodeposition current simultaneously one can control the length of the nanowires accurately and have a full knowledge of current efficiency.

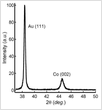
A pH of 6.0 is important to maintain is needed to get the preferred crystalline orientation of the cobalt nanowires. This of course would differ for other materials.
- P(S-b-MMA) block copolymer as described in this process (link to BCP process)
- Quartz crystal microbalance
- Saturated calomel electrode (SCE), reference electrode
- Platinum foil, counter electrode
- Electrolyte consisting of deionized water, methanol (surfactant, CH3OH, 20% by volume), cobalt sulfate (CoSO4·7H2O, 1 M), and boric acid (H3BO3, 0.6 M)
- Sodium tetraborate for pH adjustment (Na2B4O7·10H2O, 0.1 M)
Clean room environment
Maxtek RQCM
